PREPARATION DE L'ASPIRINE
(texte anglais ŕ traduire, extrait du Vögel)
Phenols, unlike amines, cannot be acetylated
satisfactorily in aqueous solution: acetylation proceeds
readily with acetic anhydride in the presence of a little concentrated sulphuric acid as catalyst. Salicylic acid (o-hydroxy-benzoďc acid)upon acetylation yields
acetylsalicylic acid or aspirin:
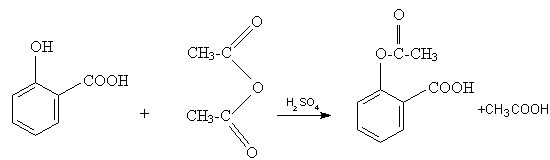
Place 10g. of dry salicylic acid and 15g.
(14mL) of acetic anhydride in a small conical flask, add 5 drops of
concentrated sulphuric acid, and rotate the flask in
order to secure thorough mixing. Warm on a water bath to about 50-60°C,
stirring with the thermometer, for about 15 minutes. Allow the mixture to cool
and stir occasionally. Add 150 mL of water, stir well and filter at the pump. Recrystallise
the crude acetylsalicylic acid from a mixture of equal volumes of acetic acid
and water.
The following is an alternative method of purifying the crude aspirin. Dissolve
the solid in about 30 mL of hot alcohol and pour the
solution into about 75 mL of warm water: if a solid
separates at this point, warm the mixture until solution is complete and then
allow the clear solution to cool slowly. Beautiful needle-like crystals will
separate. The yield is 13g. The air-dried crude product may also be recrystallised from benzene or from ether-light petroleum(b.p. 40-60°C)
Acetylsalicylic acid decomposes when heated and does not possess a true,
clearly-defined m.p. Decomposition points ranging
from 128°C to 135°C have been recorded; a value of 129-133°C is obtained on a
electric hot plate. Some decomposition may occur if the compound is recrystallised from a solvent of high boilng
point or if the boiling period during recrystallisation
is unduly prolonged.